Localised, solid cancers rely on a supply of oxygen and nutrition provided by the tumour microenvironment - the cluster of normal cells, molecules, and blood vessels that surrounds the tumour, but is not part of it. The blood supply of a tumour is chaotic and when it develops rapidly, it can outgrow its own oxygen supplies. The oxygen-starved cancer cells that result, while still deadly, resist both RT and chemotherapy. Most frustratingly, the RT-resistant, oxygen-starved, or hypoxic, regions are not static - they change and move in time. However, RT is currently delivered in regular, scheduled sessions, to the same volume of tissue, containing the tumour, as determined by an accurate snapshot in time from a CT or MRI image. At the same time, advanced RT technology now enables higher doses of X-ray treatment to be aimed selectively at different parts of a cancer. This creates an urgent, unmet clinical priority: to capture in space and time the rapid, highly-localised and transitory changes in hypoxia, pH and other key biomarkers (eg proteins and nucleic acids) that influence a tumour's local response to RT and chemotherapy. Hypoxia can be measured indirectly on tumour biopsies and by imaging or directly by invasive probes. Indirect measures do not capture vital, rapid changes in oxygenation and today's invasive probes are impractical in patients.
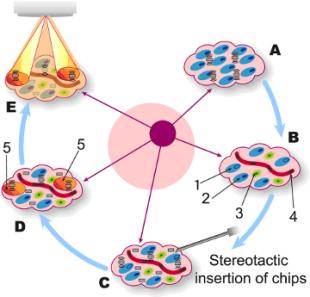
The diagram to the right shows the IMPACT principle. A group of cancer cells (A) is identified for RT treatment. This region contains RT-resistant cells (1), RT-sensitive cells (2) immune cells (3) and the blood supply (4) that form the tumour's microenvironment. Before treatment, sensor chips will be inserted stereotactically (C) amongst this cluster of cells. The sensor chips (D) will identify the RT resistant regions (5) and relay this information to the radiotherapist. RT will then be aimed and timed (E) to do maximal damage to the stubborn, RT-resistant cancer cells. IMPACT's sensors will measure not only oxygen levels, but more detailed biomarkers that indicate both the status of the tumour and the success of the highly focussed RT. For example, enzymes called Caspases are often referred to as the "executioners of cancer cell death", as they are present in large quantities when cancer cells rupture and die. IMPACT will measure their concentration to gather further intelligence on the success of RT in destroying the cancer. IMPACT also aims to ensure that this form of very personalised treatment is acceptable to both patients and their doctors, by consulting both groups and taking their views into account directly and in detail as the sensor-chip technology is developed. The IMPACT team aims to deliver 'proof of principle' in 2-3 years from now - then they will move the technology rapidly toward the clinic.